LUPIN CALIFORNIA NOTICE AT COLLECTION: SOLOSEC
Effective Date: 03/12/2024
Lupin Pharmaceuticals, Inc. ("Company," "Lupin," "we", "us," or "our") respects your privacy. This California Notice at Collection ("Notice") provides you with timely notice before or at the time when we collect Personal Information from you. In this Notice, we explain the categories and sources of Personal Information we collect from you and how we use, share, and protect that Personal Information. This Notice applies to Personal Information you provide whether your visit our Solosec product websites at www.solosec.com and www.solosechcp.com (together, "Website"), or interact with us over the phone, or email.
Please visit our Privacy Statement for more information on our privacy practices and specific provisions for California and Connecticut residents.
If you are a California resident and would like to submit a request to opt out of the selling or sharing of your Personal Information, please see the Your Privacy Choices webpage.
WE MAY MODIFY THIS NOTICE AT ANY TIME. ALL CHANGES WILL BE EFFECTIVE IMMEDIATELY UPON POSTING TO THE WEBSITE.
IF YOU PROVIDE PERSONAL INFORMATION WITH REGARD TO OUR PHARMACOVIGILANCE PROGRAM, PLEASE SEE OUR PHARMACOVIGILANCE PRIVACY STATEMENT WHICH DESCRIBES HOW WE COLLECT, USE AND SHARE THE PERSONAL INFORMATION YOU PROVIDE IN CONNECTION WITH THAT PROGRAM.
IF YOU ARE A WASHINGTON OR NEVADA RESIDENT, PLEASE SEE OUR CONSUMER HEALTH PRIVACY STATEMENT WHICH DESCRIBES HOW WE COLLECT, USE, AND SHARE THE PERSONAL INFORMATION YOU PROVIDE IN CONNECTION WITH YOUR HEALTH STATUS.
1. WHAT IS PERSONAL INFORMATION?
"Personal Information" is information that identifies, relates to, describes, is reasonably capable of being associated with, or could reasonably be linked, directly or indirectly, with you or your household. Personal Information does not include publicly available information or aggregated information that does not include personal identifiers.
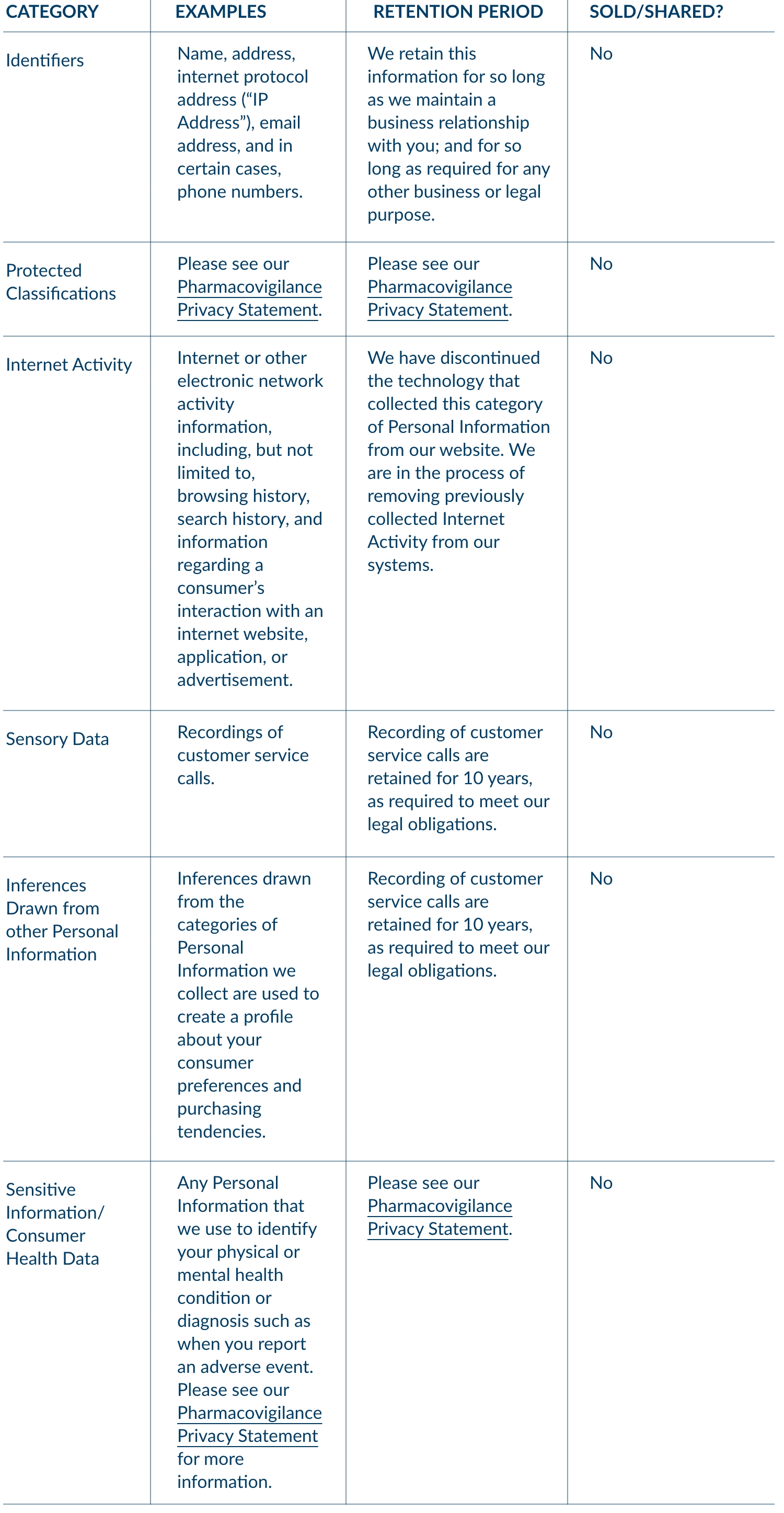
2. CATEGORIES OF PERSONAL INFORMATION
We may collect the following categories of your Personal Information. We have collected the following categories of Personal Information from consumers within the twelve (12) months preceding the effective date of this Privacy Statement:
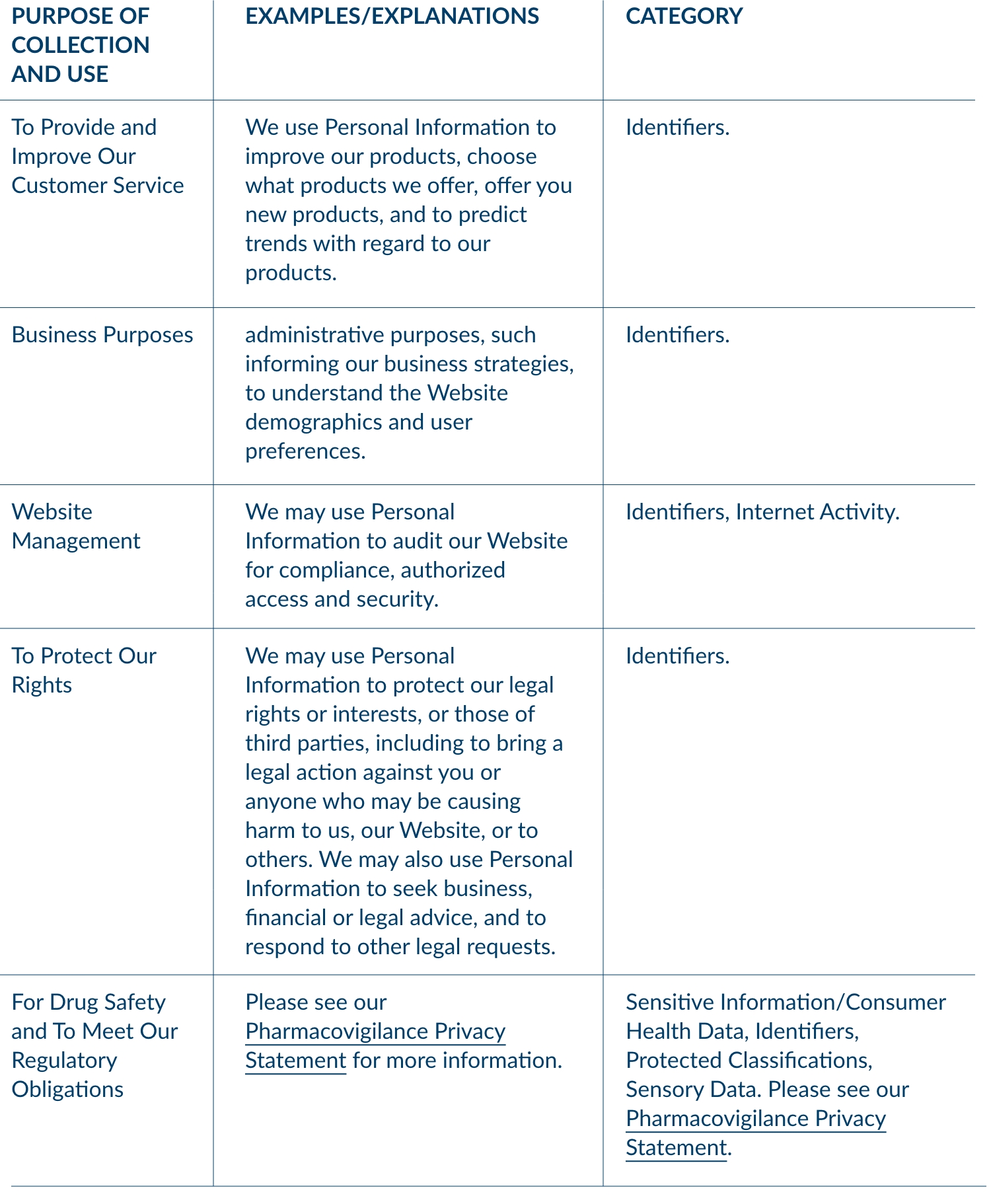
3. WHAT ARE THE PURPOSES FOR WHICH WE COLLECT PERSONAL INFORMATION?
We collect Personal Information for the following business or commercial purposes: